By using diaphragm seals, pressure measuring instruments can be adapted to even the harshest of process conditions. Here, a diaphragm separates the measuring instrument from the process medium and transmits the pressure hydraulically. However, even a diaphragm made from stainless steel can wear over time if it is permanently exposed to harsh conditions. A double-diaphragm design offers a solution for pharmaceutical applications, where the process must remain sealed under all circumstances and the hazard risk for people and the environment must be minimised.
One of the main goals of the European Union is consumer protection and the welfare of its citizens. Derived from this goal, the community of states has set up extensive rules for producers of consumer goods. Among these goods are drugs and active substances used for recovery and staying healthy. The GMP (Good Manufacturing Practice) guidelines apply, in particular, for pharmaceutical production. Good Manufacturing Practice essentially means that all foreseen measures which guarantee that the drug has the required quality for the intended use are taken.
From the wide variety of steps within pharmaceutical production, preventing the contamination of the medicines is, among others, a major aspect. This applies both to the ingredients and to the equipment with which the medications are manufactured. The plant equipment includes the measurement instrumentation, which for many production steps delivers important data on the quality of the production process – for example for the measured variable 'pressure'.
Critical media are used in many processes of the pharmaceutical industry. To guarantee that the measuring instruments are easy to clean and a precise measurement can be provided reliably, the manufacturers use diaphragm seal systems.
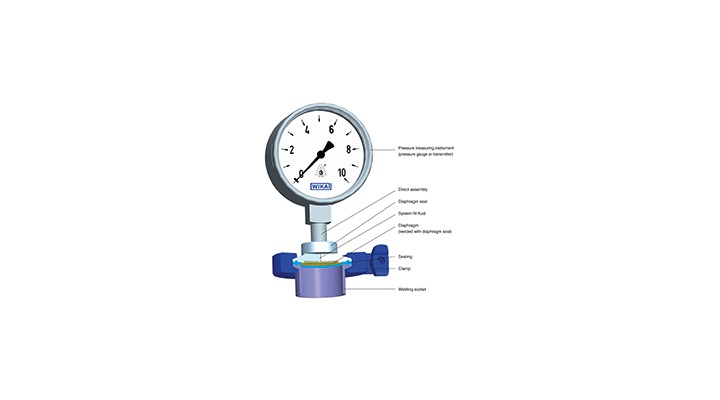
These systems are a combination of a measuring instrument, the diaphragm seal itself and the system fill fluid. An elastic, corrosion-resistant metal diaphragm shields the measuring instrument from the medium. The space between the diaphragm and the measuring instrument is completely filled with a fill fluid. Its nature (for example glycerine or paraffin oil) depends on the measuring task at hand. The diaphragm takes the process pressure and hydraulically transmits it to the measuring instrument, such as a pressure gauge, transmitter or pressure switch.
All wetted parts are, as standard, from stainless steel. Anyone demanding greater resistance against corrosion can fall back on variants from nickel-based alloys such as Hastelloy C276.
Diaphragm seals are either mounted directly to the measuring instrument or connected to it via a flexible capillary. For production processes with high temperatures a cooling element can be mounted in-between. Even in the harshest of process conditions such diaphragm seal systems measure the pressure reliably.
However, one risk remains: Very severe operating conditions can cause an unforeseen process disturbance, damaging or even destroying the sensitive diaphragm. In such a case, the system fill fluid from the diaphragm seal can find its way into the process. As a result, care must be taken when specifying the fluid for sanitary applications, and one must ensure that it is suitable for contact with the particular medium. This can be documented through conformity with the provisions of the American Food and Drug Administration (FDA). For compliance with the GMP guidelines further supporting documents are required, for example a listing in the country-specific pharmacopoeia such as the EP (European Pharmacopoeia) or USP (US Pharmacopeia) for North America.
However, these tolerances are not at all unlimited: In a number of processes in the pharmaceutical industry, contamination must be prevented under all circumstances – in any direction. Thus no system fill fluid may be allowed to enter the product, in order to protect the purity of the pharmaceutical material. At the same time, the risk of dangerous substances escaping into the environment must be prevented. This is the case, for example, when producing vaccines with live viruses or of genetically modified organisms. The consequences would be dramatic in each case. The safety of the consumers, the plant workers and the environment is therefore the top priority.
For anyone who must exclude all eventualities, a more advanced diaphragm seal system from WIKA is available: with a double diaphragm and diaphragm break monitoring. Using this patented system, the space between the two diaphragms is evacuated and the vacuum can be monitored with a measuring instrument. The type of monitoring can be specified individually, depending on the sensitivity of the process. With regular on-site inspection, for example, a pressure gauge with green-red display will be sufficient, in other cases a visual or audible alarm in the control room may be required. When using media with a high risk potential, the operator can use a pressure switch which will immediately halt the process in the event of any damage.
Should the wetted diaphragm become damaged as a result of extreme loading or due to an aggressive medium, the second forms a reliable seal to the process and maintains the pressure monitoring until the damage has been rectified. Since a break within the system can be detected and reported immediately, no microbes can get past the diaphragm undetected.
For pharmaceutical applications, the comprehensive process safety is the most important feature of the diaphragm seal systems with double diaphragm. Furthermore, these systems provide the plant operators with a significant time advantage: When utilising instruments with "simple" diaphragm seals they must, often after each batch, remove all measuring instruments from the process and check the diaphragms for possible damage. Only then can the product batch be released for further processing. This operation and the waiting time before release are dispensed with when measuring assemblies with integrated diaphragm break monitoring are used.
Conclusion
Investing in the safety of pharmaceutical processes is an inevitable task, especially with regard to the possibly severe consequences. In terms of protection of product and environment, the diaphragm seal systems described in this text offer considerable advantages. Matching them to the individual process environments does involve additional planning. However, in the long run the economic aspects of this measurement technology – the simplified process control, reduced waiting and downtime and minimised failure risk – will make up for this. In this case, improved efficiency means reducing the costs and optimising the running operation.
















-205x205.jpg)